FACILITY
Good Tissue Practice (GTP) Human Cell and Tissue Processing Room
In May 2005, the United States officially implemented the basic requirements for regulations on “human cells, tissues, and cellular and tissue-based products (HCT/Ps)”. The development of treatment-related products must comply with the cell tissue laboratory specifications of GTP (Good Tissue Practice). Mariavon GTP cell tissue laboratory has passed the inspection of the Department of Health in 2010.
Air conditioning system :Experimental area pressure difference design + 3-channel air filtration system, dynamically reducing the probability of contamination through air flow, maintaining a sterile and dust-free environment.
Central computer environment monitoring:Strictly control the entry and exit of personnel through door cards and video surveillance systems, strictly monitor the laboratory environment, and maintain constant temperature, constant humidity, and constant pressure.
Strict quality control of experimental reagents and cell preparations :sterility test/ mycoplasma test/ endotoxin test/ cell antibody staining.
GTP Laboratory Floor Plan
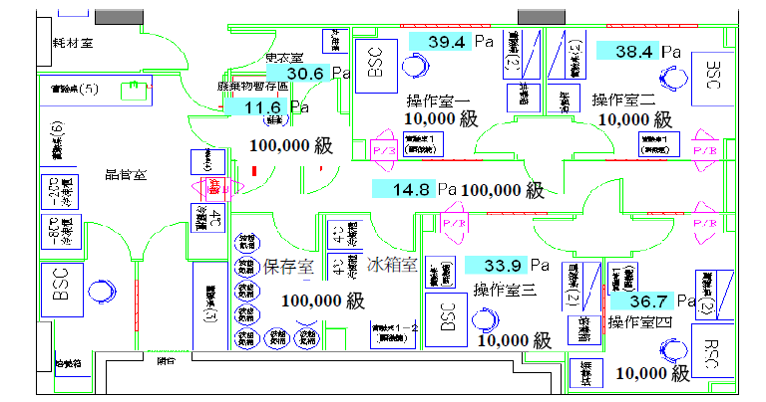
GTP Laboratory Floor Plan

Cell Culture Operation Room

Quality Control Room

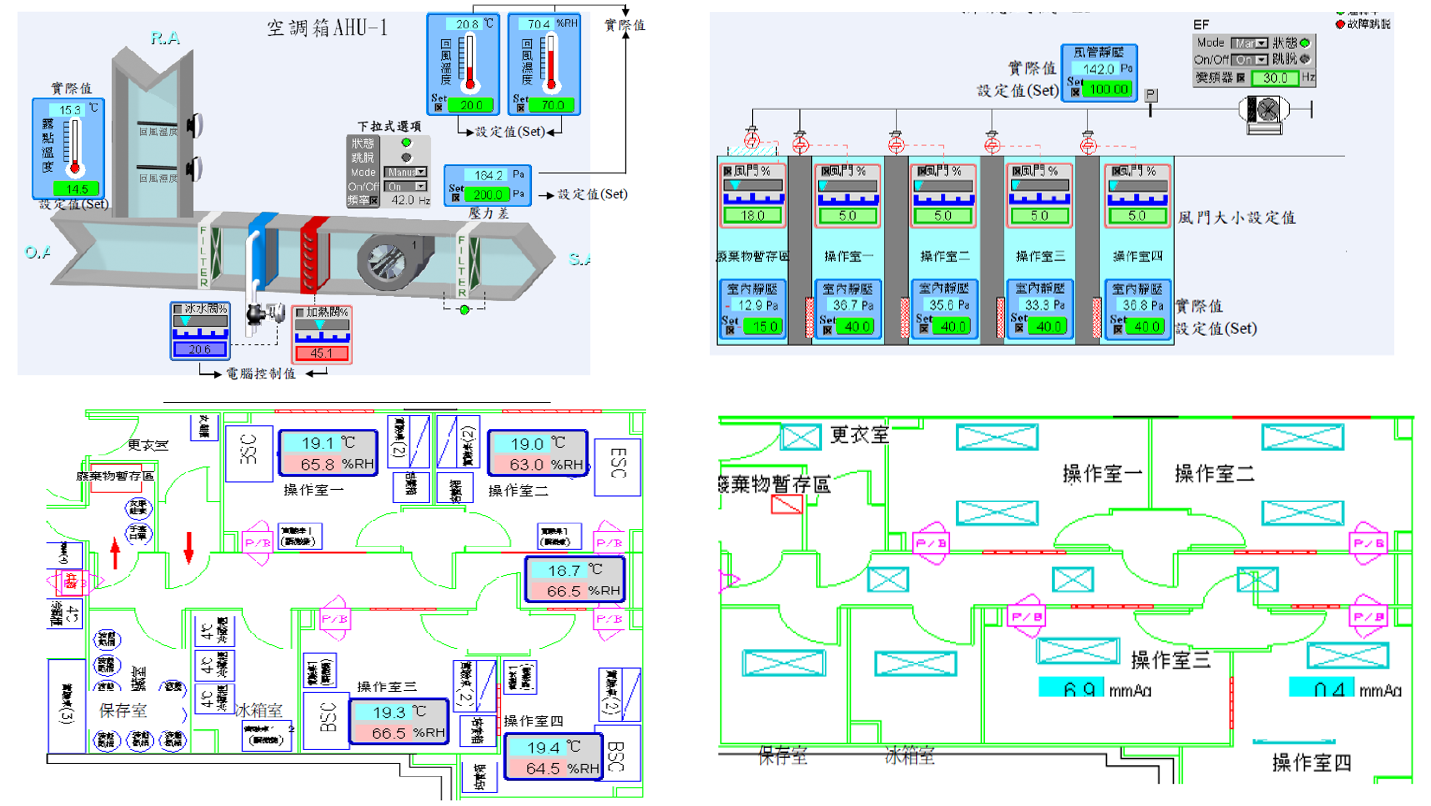
GTP Laboratory Environmental Monitoring System
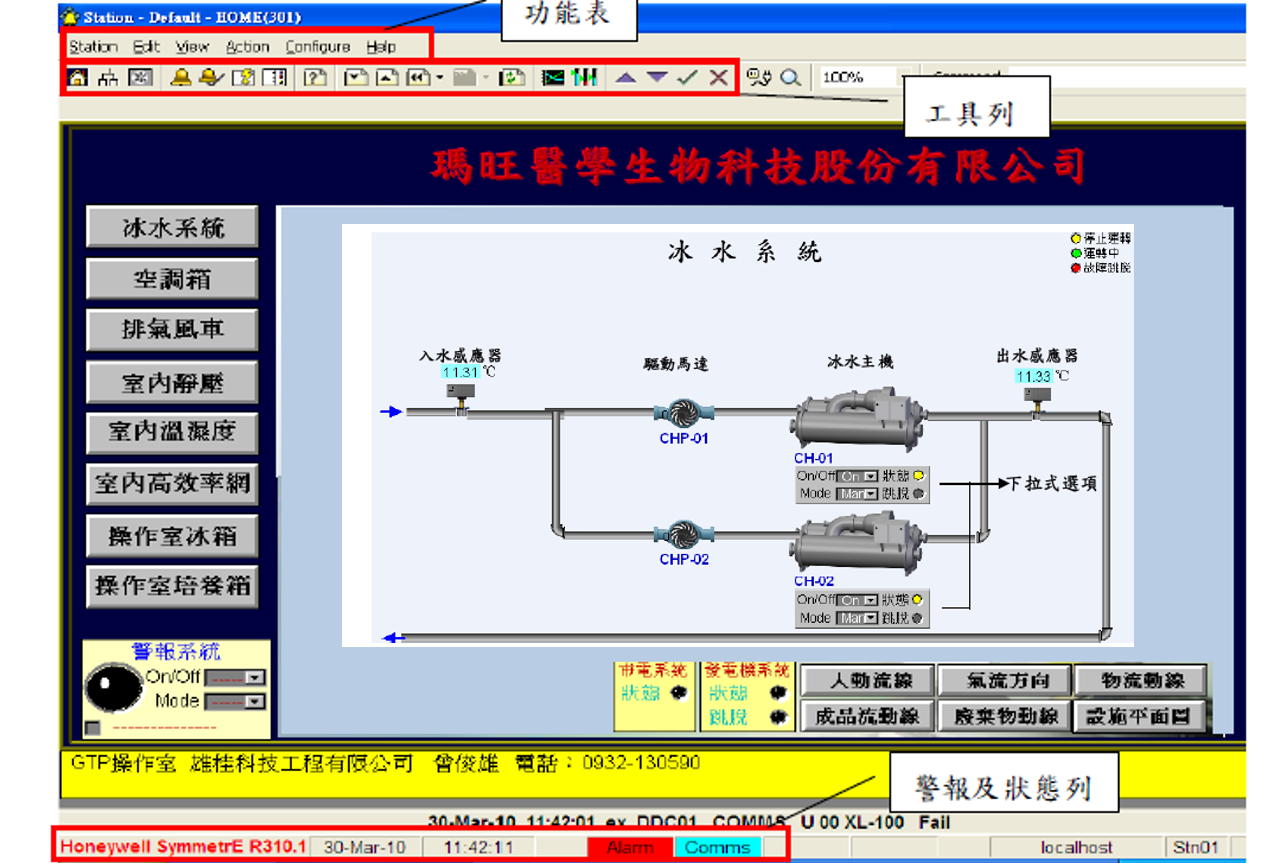
GTP Laboratory Temperature, Humidity, and Pressure Monitoring System
Sterility Testing / Endotoxin / Mycoplasma Testing Reports

COMPANY INFORMATION
Address: 7th Floor, No. 29-1, Lane 169, Kangning Street, Xizhi District, New Taipei City
Phone:+886-2-2693-3232

